We wanted to wish Yolanda Ciuro a Happy Birthday today! We hope your day is full of joy!
We wanted to wish Yolanda Ciuro a Happy Birthday today! We hope your day is full of joy!
 4
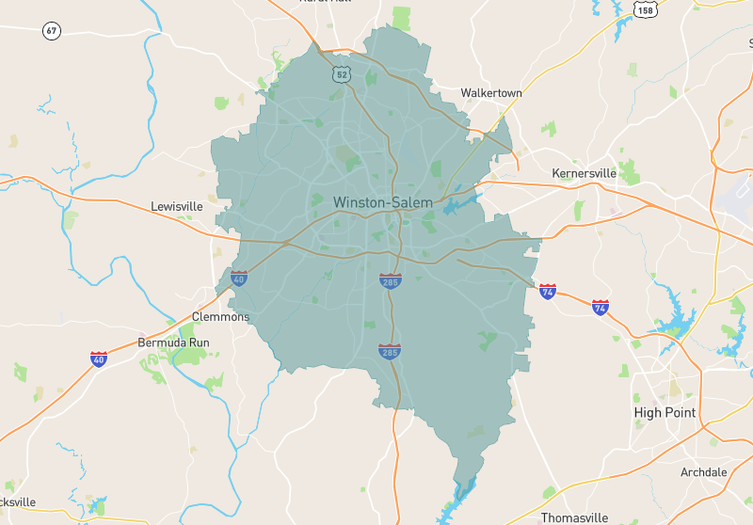
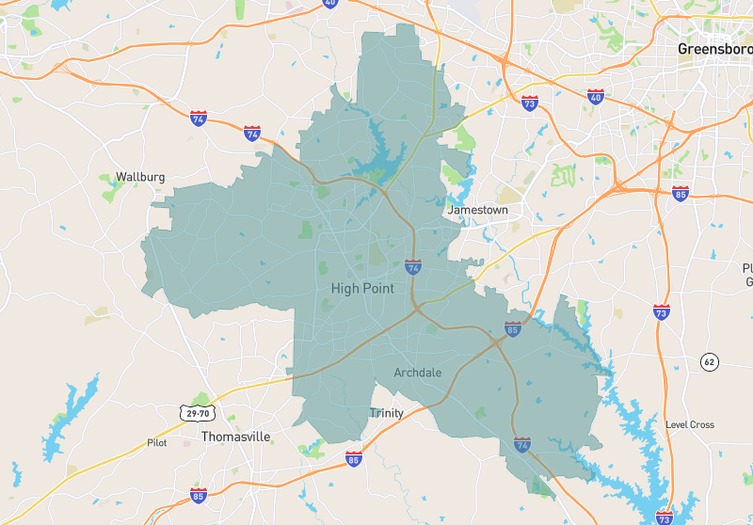
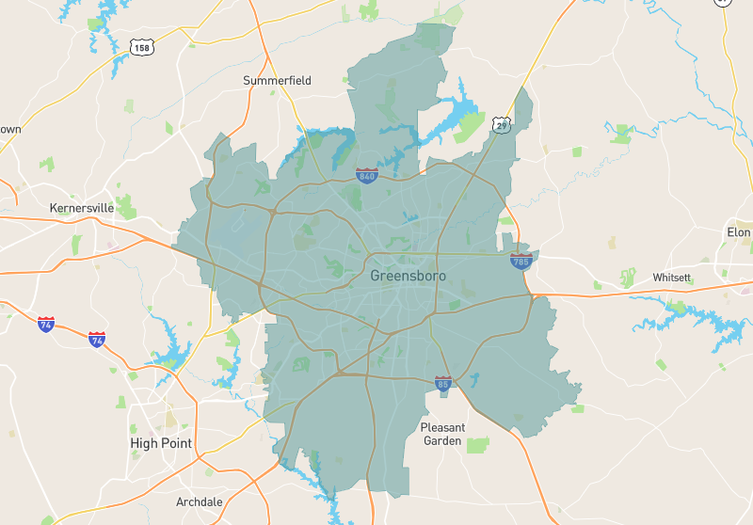
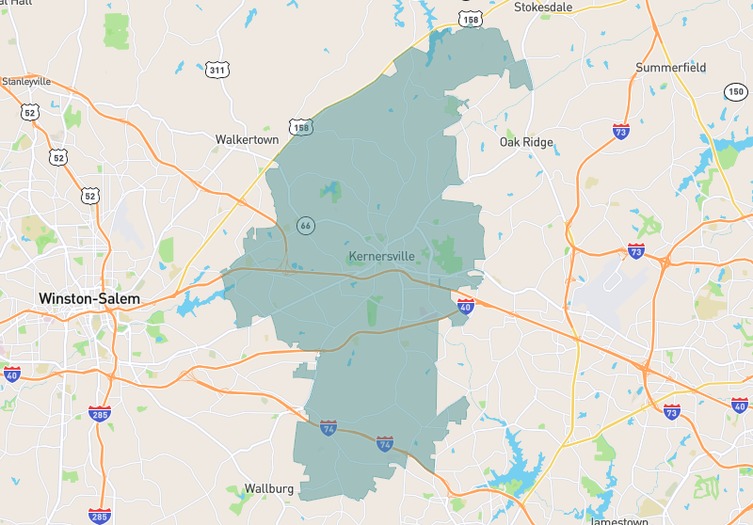
Navigating both buying and selling in Forsyth County, North Carolina, as we head into December 2025, offers a unique opportunity, thanks to evolving market dynamics. With a 2.52 months supply of inventory, we've seen a 15% increase over the past year, though the past month shows an 11% drop, creating tighter conditions…
4
Navigating both buying and selling in Forsyth County, North Carolina, as we head into December 2025, offers a unique opportunity, thanks to evolving market dynamics. With a 2.52 months supply of inventory, we've seen a 15% increase over the past year, though the past month shows an 11% drop, creating tighter conditions…
 2
We wanted to wish Kevin Cowan a Happy Birthday today! We hope your day is full of joy!
2
We wanted to wish Kevin Cowan a Happy Birthday today! We hope your day is full of joy!
 3
Cheers to fresh starts and new possibilities!
From all of us at Wayfinder, we wish you a happy, healthy, and hopeful New Year. Here’s to what’s next 🥂🏡
#wayfinderhomesllc #findyourwayhome #letushelp!
3
Cheers to fresh starts and new possibilities!
From all of us at Wayfinder, we wish you a happy, healthy, and hopeful New Year. Here’s to what’s next 🥂🏡
#wayfinderhomesllc #findyourwayhome #letushelp!
 4
No better way to end the year! 🎉
Congrats to Mary Kate Foley on the sale of 3000 NE Greenway Avenue, Winston-Salem, NC, representing the seller from start to finish. A smooth closing, happy sellers, and a strong finish to 2025 👏🏡
#wayfinderhomesllc #findyourwayhome #investorfriendly #reinvestor #triadnc
4
No better way to end the year! 🎉
Congrats to Mary Kate Foley on the sale of 3000 NE Greenway Avenue, Winston-Salem, NC, representing the seller from start to finish. A smooth closing, happy sellers, and a strong finish to 2025 👏🏡
#wayfinderhomesllc #findyourwayhome #investorfriendly #reinvestor #triadnc
 1
We wanted to wish Jennifer Padilla-Olmedo a Happy Birthday today! We hope your day is full of joy!
1
We wanted to wish Jennifer Padilla-Olmedo a Happy Birthday today! We hope your day is full of joy!
 4
Wishing you a very Merry Christmas from all of us at Wayfinder.
May your day be filled with joy, comfort, and time spent with the people who make your house feel like home. ❤️🏡
#wayfinderhomesllc #findyourwayhome
4
Wishing you a very Merry Christmas from all of us at Wayfinder.
May your day be filled with joy, comfort, and time spent with the people who make your house feel like home. ❤️🏡
#wayfinderhomesllc #findyourwayhome
 4
✨ Price Improvement | 581 Clyde Drive ✨
📍 581 Clyde Drive, Winston-Salem, NC 27104
💰 Now offered at $314,900
This beautiful home just received a price improvement, making it an even better opportunity in one of Winston-Salem’s most desirable areas! 🏡
Located on a quiet street with a convenient location, 581 Clyde Dri…
4
✨ Price Improvement | 581 Clyde Drive ✨
📍 581 Clyde Drive, Winston-Salem, NC 27104
💰 Now offered at $314,900
This beautiful home just received a price improvement, making it an even better opportunity in one of Winston-Salem’s most desirable areas! 🏡
Located on a quiet street with a convenient location, 581 Clyde Dri…